📄 Full Whitepaper Available
This comprehensive 20+ page whitepaper is available as a standalone document with full academic styling, references, and technical appendices.
Read Full Whitepaper →Executive Summary
Clinical trial participants represent the foundation of pharmaceutical innovation, yet current data management practices systematically erase their contributions within months of consent expiration. This whitepaper presents GenoVault, a blockchain-secured patient data sovereignty platform that transforms clinical trial participants from transient data sources into permanent scientific partners.
The Problem: Lost Signal Patients
When rare responders, unexpected adverse events, or breakthrough discoveries emerge years after trial completion, the “signal patients” whose data could unlock the next therapeutic generation have vanished from institutional databases. Traditional workflows destroy biosamples and erase genomic data following consent expiration—a practice that has cost pharmaceutical research immeasurable scientific value.
Traditional Clinical Trial Data Flow (Data Loss Crisis)
graph TD
A[Patient Enrolls in Clinical Trial] -->|Signs Consent| B[Institution Collects Biosample]
B --> C[Genomic Sequencing]
C --> D[Data Stored in Institutional Database]
D --> E[Trial Analysis Completed]
E --> F{Consent Expires?}
F -->|Yes - 12-24 months| G[DELETE: Biosample Destroyed]
G --> H[DELETE: Genomic Data Purged]
H --> I[Patient Lost to Follow-Up]
J[Years Later: Breakthrough Discovery] -.->|Need Signal Patient Data| I
I -.->|DATA PERMANENTLY LOST| K[❌ Cannot Validate Biomarker]
K -.-> L[❌ Delayed Drug Development]
L -.-> M[$300-500M NPV Loss]
style G fill:#ff6b6b,stroke:#c92a2a,color:#fff
style H fill:#ff6b6b,stroke:#c92a2a,color:#fff
style K fill:#ff6b6b,stroke:#c92a2a,color:#fff
style L fill:#ff6b6b,stroke:#c92a2a,color:#fff
style M fill:#ff6b6b,stroke:#c92a2a,color:#fff
Critical Failure Points:
- Consent Expiration = Mandatory data deletion (HIPAA/IRB requirements)
- Institutional Custody = Patient loses control after trial ends
- No Recontact Mechanism = Cannot locate patients years later
- Permanent Loss = Exceptional responder data vanishes forever
The Solution: Patient-Owned GenoVault
GenoVault implements patient-controlled blockchain infrastructure that:
- Preserves Data Integrity Across Decades: Patient-owned (or institution-mirrored) genomic data vaults maintain data for generations
- Enables Granular Consent: BioNFT-based access control allows patients to grant/revoke permissions in real-time without legal intermediaries
- Supports Cross-Border Collaboration: x402 BioData Router enables cross-lab, cross-border, cross-program data sharing
- Ensures Attribution & Economic Participation: Story Protocol PIL integration provides immutable attribution and royalty sharing
GenoVault Clinical Trial Data Flow (Patient Sovereignty Model)
graph TD
A[Patient Creates Blockchain Wallet] -->|Permanent Identity| B[Enrolls in Clinical Trial]
B --> C[Institution Performs Sequencing]
C --> D[Data Uploaded to Patient GenoVault]
D --> E[Patient Mints BioNFT]
E -->|Grants Access| F[Trial Sponsor Receives Cryptographic Permissions]
F --> G[Trial Analysis Completed]
G --> H{Trial Ends}
H -->|BioNFT Expires| I[Patient Retains Data in Vault]
I --> J[Patient Maintains Permanent Ownership]
K[Years Later: Breakthrough Discovery] -->|Blockchain Query| J
J -->|Patient Still Accessible| L[Sponsor Requests Recontact]
L --> M[Patient Reviews New Protocol]
M -->|Consents| N[Mints New BioNFT]
N --> O[✅ Instant Data Access]
O --> P[✅ Biomarker Validated in Weeks]
P --> Q[✅ Accelerated Diagnostic Approval]
Q --> R[$300-500M NPV Captured]
R --> S[Patient Receives 2-5% Royalty Share]
style I fill:#51cf66,stroke:#2f9e44,color:#000
style J fill:#51cf66,stroke:#2f9e44,color:#000
style O fill:#51cf66,stroke:#2f9e44,color:#000
style P fill:#51cf66,stroke:#2f9e44,color:#000
style Q fill:#51cf66,stroke:#2f9e44,color:#000
style S fill:#ffd43b,stroke:#fab005,color:#000
Success Enablers:
- Permanent Wallet Identity = Patient never “lost to follow-up”
- Patient Control = Blockchain-verified cryptographic permissions
- Renewable Consent = Patient mints new BioNFTs for future studies
- Economic Participation = Story Protocol PIL royalty sharing ($5,400/patient/year from successful biomarkers)
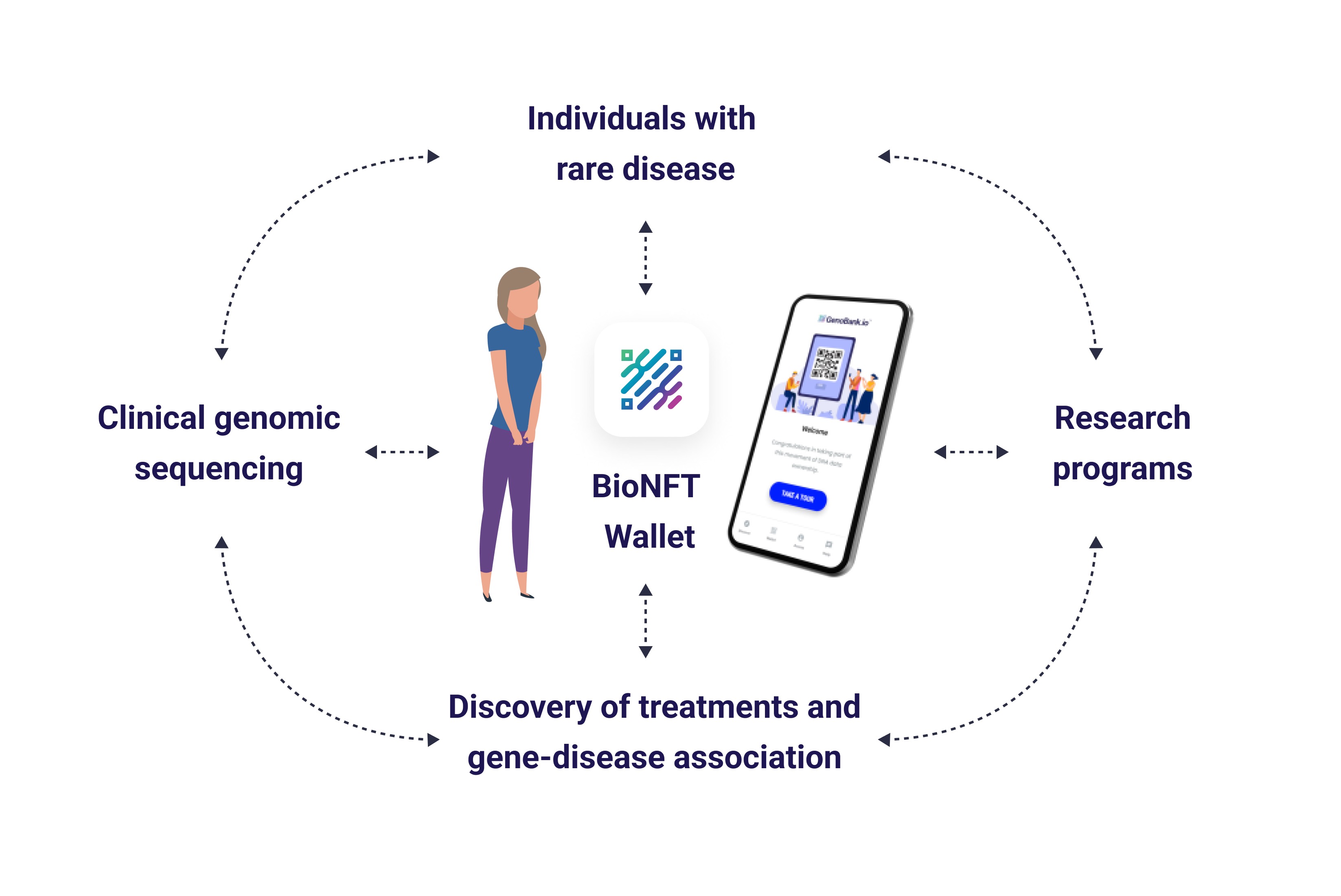
Figure 1: Rare Disease Patient Data Cycle - Circular flow connecting individuals with rare disease, clinical genomic sequencing, BioNFT Wallet, research programs, and discovery of treatments and gene-disease associations. The patient-controlled wallet maintains permanent data sovereignty across all research phases.
Web Evolution: From Institutional Custody to Patient Sovereignty
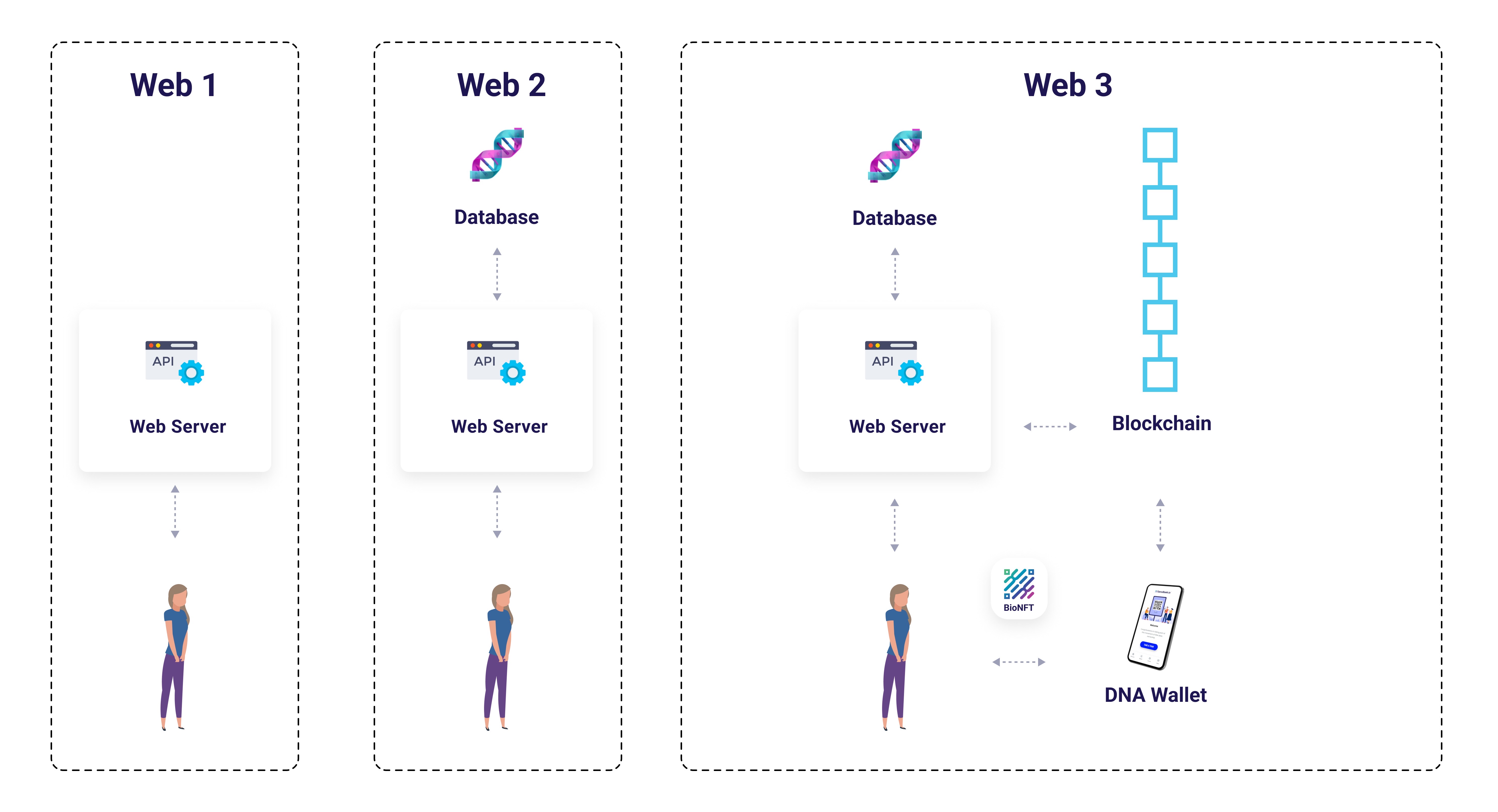
Figure 2: Web Evolution - Progression from centralized institutional databases (Web1/Web2) to blockchain-based patient ownership (Web3) with BioNFT and DNA Wallet. Traditional models concentrate data control in institutional servers; Web3 returns sovereignty to patients via cryptographic access control.
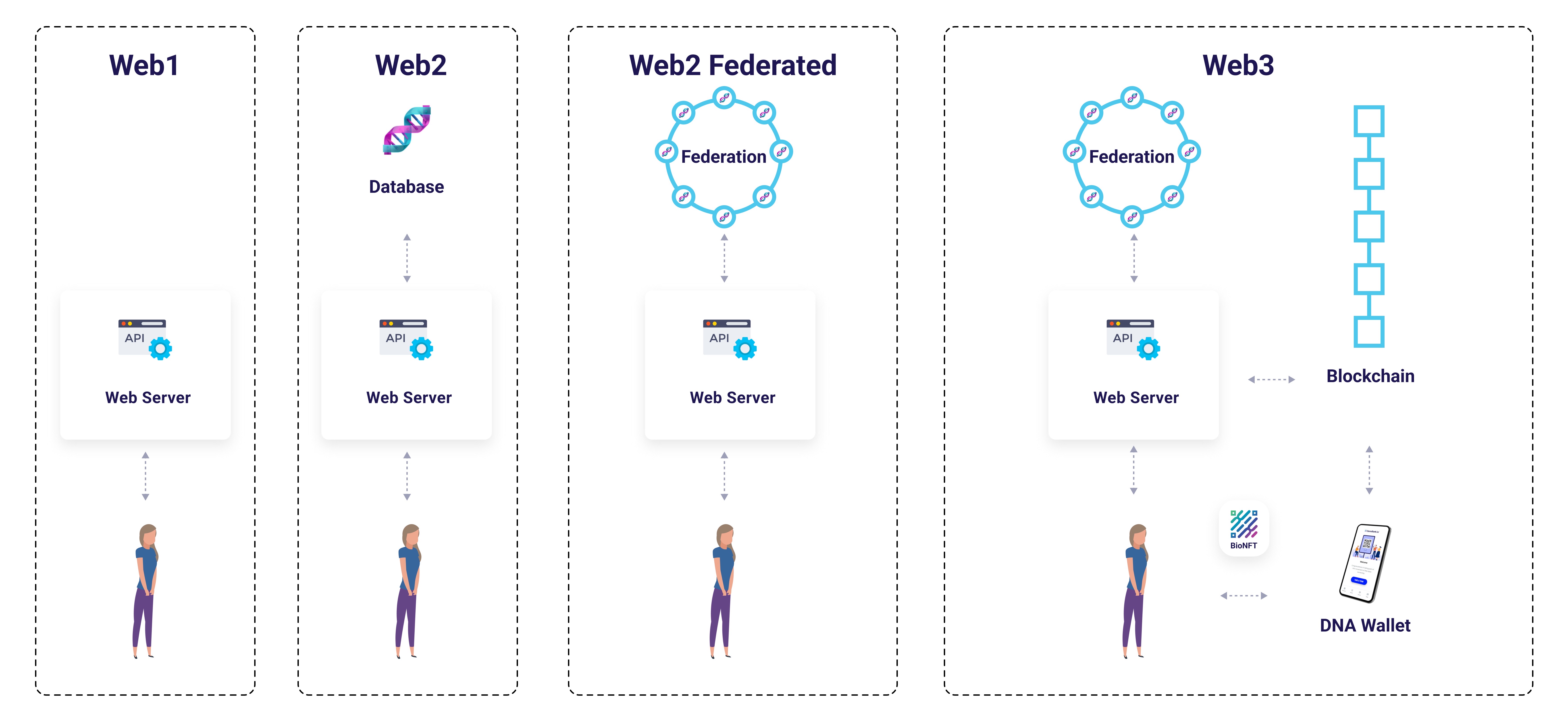
Figure 3: Web2 Federated vs Web3 Architecture - Evolution showing federated data networks (Web2 Federated) and blockchain-secured patient control (Web3). GenoVault implements Web3 architecture with BioFS Protocol federation, combining institutional operational expertise with patient cryptographic sovereignty.
Real-World Impact: HER2+ Breast Cancer & Enhertu Development
Using DESTINY-Breast clinical trials for Enhertu (trastuzumab deruxtecan) as a case study, this whitepaper demonstrates:
- Accelerated Companion Diagnostic Development: 18-24 month reduction in biomarker validation timelines worth $300-500M NPV
- Reduced Clinical Trial Costs: 51-77% cost reduction ($814 vs. $2,500-3,500 per analysis)
- Faster Recruitment: 30% improvement through economic participation incentives
- Improved Retention: 50% reduction in loss-to-follow-up through patient sovereignty model
Technical Architecture
BioFS Protocol (Blockchain-Integrated Federated Storage):
- Privacy-preserving DNA fingerprints using SHA-256 cryptographic indexing
- 42 laboratories, 8,547 indexed genomic samples
- <100ms query latency, zero gas cost for discovery
- GDPR-compliant through control/data plane separation
x402 BioData Router:
- ERC-8004 non-transferable BioNFT consent tokens
- EIP-3009 gasless patient experience
- Atomic multi-party payment settlement
- 47 completed international whole exome analyses
Story Protocol PIL Integration:
- Programmable IP licensing for genomic contributions
- Automatic royalty distribution (2% diagnostic revenue + 0.5% pharmaceutical premium)
- Estimated $5,400/patient/year from successful biomarker discoveries
Side-by-Side Comparison: Traditional vs GenoVault
graph LR
subgraph Traditional["❌ Traditional Model"]
direction TB
T1[Patient Enrolls] --> T2[Institution Owns Data]
T2 --> T3[Trial Ends]
T3 --> T4[Consent Expires]
T4 --> T5[Data Deleted]
T5 --> T6[Patient Lost]
T6 -.->|Cannot Recontact| T7[Discovery Impossible]
end
subgraph GenoVault["✅ GenoVault Model"]
direction TB
G1[Patient Creates Wallet] --> G2[Patient Owns Data]
G2 --> G3[Trial Ends]
G3 --> G4[BioNFT Expires]
G4 --> G5[Data Preserved]
G5 --> G6[Patient Accessible]
G6 -->|Blockchain Query| G7[Discovery Enabled]
G7 --> G8[Patient Earns Royalties]
end
style T5 fill:#ff6b6b,stroke:#c92a2a
style T6 fill:#ff6b6b,stroke:#c92a2a
style T7 fill:#ff6b6b,stroke:#c92a2a
style G5 fill:#51cf66,stroke:#2f9e44
style G6 fill:#51cf66,stroke:#2f9e44
style G7 fill:#51cf66,stroke:#2f9e44
style G8 fill:#ffd43b,stroke:#fab005
Key Differentiators:
- Data Custody: Institutional (deleted after trial) vs. Patient-Owned (permanent)
- Recontact Ability: Lost after consent expires vs. Always accessible via blockchain
- Economic Model: Zero patient compensation vs. 2-5% royalty sharing
- Timeline: Biomarker validation delayed by years vs. Completed in weeks
Key Sections
1. The Clinical Trial Data Loss Crisis
The 23andMe bankruptcy (2025) illustrated catastrophic failure of centralized genomic data custody—15 million customers had zero say in the bankruptcy sale of their genomic data. This proves that policy-based privacy protections fail when institutional control overrides patient autonomy.
GenoVault’s Patient Sovereignty Model Ensures:
- Pharmaceutical company bankruptcy cannot transfer patient data (patients retain cryptographic access control)
- Institutional ownership changes require no patient action (BioNFT permissions persist)
- Cross-border regulatory changes don’t orphan patient data (patient wallets transcend jurisdictions)
2. Economic Impact: The Value Attribution Gap
Current Model: Patients receive $0 compensation when their genomic data enables companion diagnostic approvals worth $200M-1B+ annually.
GenoVault Model: Story Protocol PIL revenue sharing:
- 2% of diagnostic test revenue ($1.2M/year ÷ 500 patients = $2,400/patient/year)
- 0.5% of pharmaceutical pricing premium ($1.5M/year ÷ 500 patients = $3,000/patient/year)
- Total: $5,400/patient/year + potential $10,000-50,000 cumulative royalties for exceptional responders
3. Cross-Border Clinical Trial Coordination
Traditional Approach:
- 18 institutional data transfer agreements required
- 6-month legal setup timeline
- $2.5M legal fees
- 4-8 weeks per cross-site data access request
GenoVault Approach:
- LabNFT credential verification (one-time setup)
- 2-week legal setup
- $50K total costs
- 5 seconds for BioNFT validation
4. Privacy, Security & Regulatory Compliance
GDPR Article 17 Compliance:
- Control plane (immutable blockchain): Pseudonymous wallet addresses, DNA fingerprints, audit trails
- Data plane (deletable off-chain): VCF files, BAM sequences, clinical phenotypes
- Patient exercises right to erasure by burning BioNFTs and deleting S3 files
HIPAA Compliance:
- Safe Harbor Method: Blockchain records contain only pseudonymous wallet addresses
- Limited Dataset with Data Use Agreement: Genomic files shared under BioNFT authorization
- Audit Trail: Blockchain transaction history provides tamper-proof breach notification evidence
Security Architecture:
- 15-minute presigned S3 URL expiration
- Client-side AES-256 encryption
- Multi-signature smart contract upgrades
- Social recovery mechanisms for key loss
Implementation Roadmap
Phase 1 (Months 1-6): Technical infrastructure deployment
- Deploy BiodataRouter and LabNFT smart contracts
- Partner with 3-5 CLIA-certified laboratories
- Success Metric: 5 LabNFTs, 100 test patient wallets
Phase 2 (Months 7-12): Limited enrollment clinical study
- Recruit 50 HER2+ breast cancer patients into observational registry
- Validate BioNFT-gated access control
- Success Metric: 50 patients enrolled, >80% satisfaction
Phase 3 (Months 13-24): Pharmaceutical partnership pilot
- Partner with AstraZeneca DESTINY-series trials
- Enroll 200 patients with dual consent (traditional IRB + GenoVault BioNFT)
- Success Metric: 30% faster recruitment, 50% reduced loss-to-follow-up
Performance Metrics
| Operation | Latency | Gas Cost | USD Equivalent |
|---|---|---|---|
| LabNFT Registration | 5 seconds | 250,000 gas | $0.75 |
| DNA Fingerprint Index | 3 seconds | 80,000 gas | $0.25 |
| Fingerprint Query | <100ms | 0 gas (read-only) | $0.00 |
| BioNFT Minting | 5 seconds | 150,000 gas | $0.45 |
| Presigned URL Generation | 200ms | 0 gas (off-chain) | $0.00 |
Conclusion: A New Social Contract for Clinical Research
GenoVault proposes a fundamental reimagining of the ethical relationship between patients, institutions, and pharmaceutical innovation: patients as sovereign stakeholders maintaining permanent ownership of their contributions while enabling collaborative research through programmable consent and economic participation.
When patients retain sovereignty, receive attribution, and participate economically in derivative discoveries, clinical research becomes a collaborative partnership rather than an extractive transaction.
The question is no longer “Can blockchain enable patient data sovereignty?” but rather “How quickly can we deploy this infrastructure to prevent the next generation of lost signal patients and scientific opportunity costs?”